| Name: | Mgr. Vendula Krynická, PhD. Laboratory of Photosynthesis Josef Komenda`s group |
|---|---|
| Phone: | +420 384 340 444 |
| E-mail: | krynicka(at)alga.cz |
| Position: | Associate Scientist |
Vendula Krynická
ORCID: 0000-0002-1887-5986
Curriculum vitae
Research interest and current research work
Vendula focuses on regulatory processes during acclimation of the cyanobacterium Synechocystis PCC 6803 to various environmental stresses, including whole-cell transcriptome and proteome responses, and she is particularly interested in the role of FtsH proteases in maintaining cellular homeostasis. She contributed to the discovery that FtsHs are crucial for the function of photosystem II (PSII) under high illumination and oxidative stress, as they play a critical role in PSII biogenesis and quality control and thus contribute significantly to photosystem robustness (Bečková et al., 2017; Krynická et al., 2023; Konik et al., 2024). She also showed that the essential FtsH1/3 complex plays a central role in the transcriptional response to nutrient deficiency. By regulating the level of a corresponding transcription factor, it maintains cellular homeostasis during iron, phosphate, carbon and nitrogen starvation in Synechocystis (Krynická et al., 2019). Vendula is currently studying the regulatory processes governing the NtcA pathway and the involvement of FtsH1/3 in its regulation. She is investigating new methods to reprogram this pathway in Synechocystis for potential use in biotechnology. To achieve this goal, she is expanding her expertise in protein modelling.
Vendula is currently leading a research group within the Photomachines project (here and here) and is one of its coordinators. She is also the principal investigator of the project Reprogramming the NtcA global regulatory pathway in cyanobacteria (GACR, 2025 - 2027).
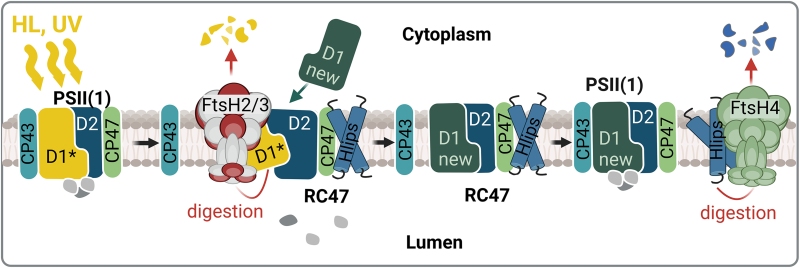
The role of the thylakoid FtsHs in photoprotection of the PSII complex. Under HL and UV stress, the fast inactivation of PSII is accompanied by the damage of the D1 protein (D1*) leading to the destabilization of CP43 binding within PSII. This destabilization allows access of FtsH2/3 to the damaged D1, which is then quickly degraded and replaced by the newly synthesized D1 (new D1). Subsequently, the CP43 antenna is reconnected to the repaired RC47. This repair of PSII occurs in the presence of high-light inducible proteins (Hlips), which protect PSII intermediates from oxidative damage by quenching the energy from the excited Chl. When the PSII repair is completed, the Hlips are released from the complex and their level is regulated by the FtsH4 protease (from Krynická and Komenda, 2024)