Laboratory of Photosynthesis
Josef Komenda`s group
Biogenesis of Photosystem II
Biogenesis and quality control of the membrane photosynthetic complexes
Our group focuses on the biogenesis and maintenance of chlorophyll (Chl)-protein complexes, photosystem I (PSI) and photosystem II (PSII), embedded in the thylakoid membranes of photosynthetic organisms. The PSI and PSII represent the key molecular machines of oxygenic photosynthesis, a biochemical proces essential for development and maintenance of life on the Earth. These large membrane complexes perform light-driven primary charge separation, resulting in reduction of NADP+ and formation of a pH gradient used for ATP synthesis. Both photosystems are among the most complex enzymes in nature due to the large number of protein subunits as well as cofactors including Chls, carotenoids, heme and non-heme iron, lipids, ions and others. Thanks to advances in structural biology we know their detailed structures (Jordan et al., 2001; Umena et al., 2011), but less explored is the complex process of biogenesis requiring strict regulation and quality control.
As an experimental model, we use the glucose tolerant strain of the cyanobacterium Synechocystis PCC 6803 (Synechocystis), characterised by its fast growth rate, natural transformability and the possibility of growing on glucose if the genes necessary for photosynthesis are knocked out, making it an excellent model for the study of fundamental processes on photosynthetic membranes, which are common to cyanobacteria, algae and higher plants. We work with modern methods of molecular biology, protein biochemistry and cell imaging, and in cooperation with our partners we also use advanced structural biology.
Our research is currently supported by the projects listed here and here.
Photosystem II biogenesis
The biogenesis of PSII has been described in basic outline as the sequential association of four assembly complexes (modules), each consisting of a large Chl-binding subunit (i.e. the key reaction center proteins D1 and D2, and the internal antennae CP43 and CP47), adjacent small transmembrane subunits and accessory (assembly) factors (AFs) facilitating individual steps of the assembly (Komenda, Sobotka and Nixon, 2012; Komenda et al., 2024). The AFs are mostly step/module specific and they may be involved in stabilizing protein-protein interactions, protecting the nascent structure from premature degradation or light damage, and promoting insertion of Chl and other cofactors. For more detailed insight to our work on this topic see e.g. Komenda et al. (2012), Knoppová et al. (2014), Bečková et al. (2017), Bučinská et al. (2018), Pascual-Aznar et al. (2021), Knoppová et al. (2022).
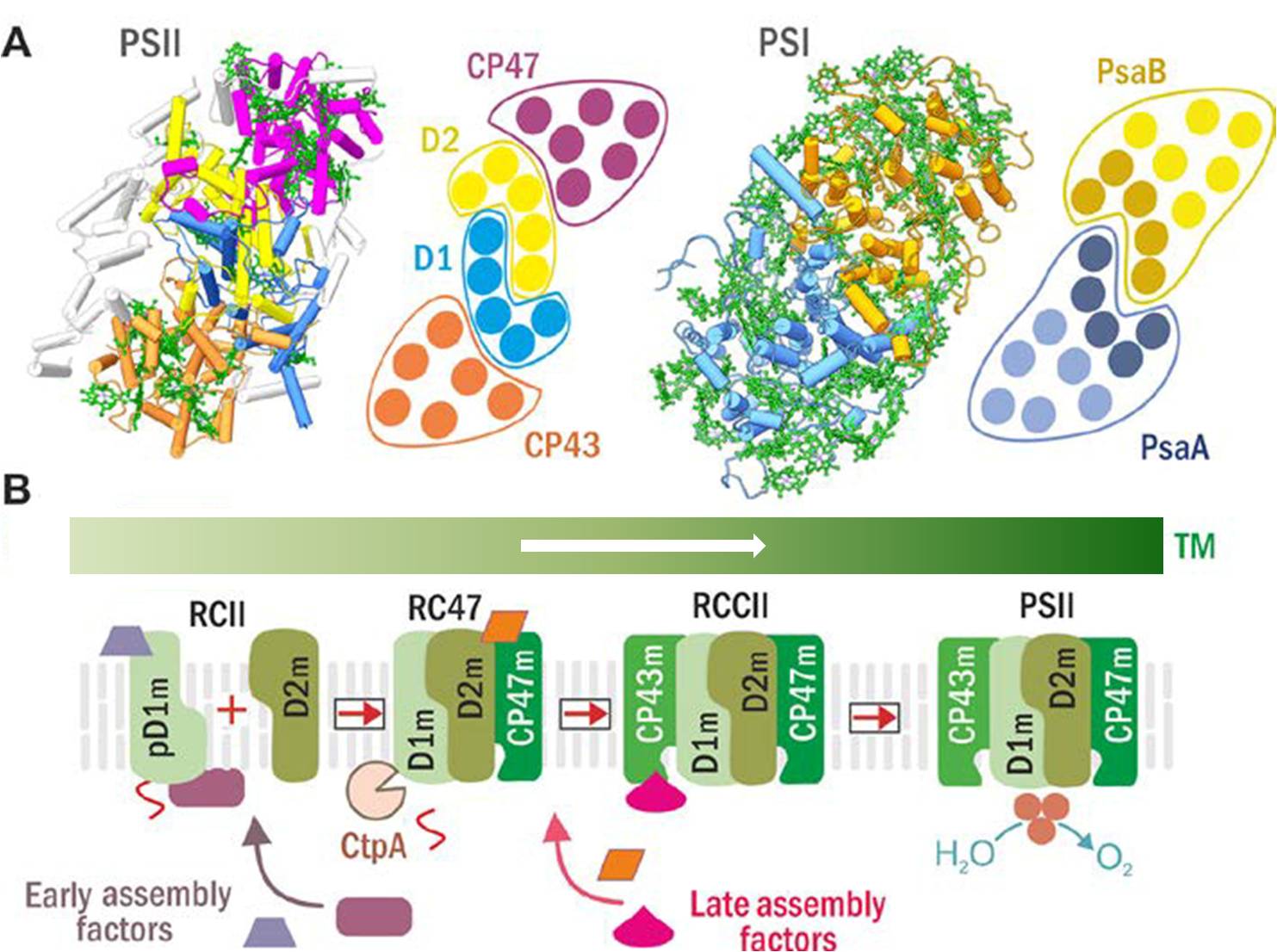
Structures of PSII and PSI (A) and scheme of the current model for PSII assembly and its spatial arrangement in cyanobacterial cells (B). (A) PSII (left) and PSI (right) structure and schematic helical arrangement of their main Chl-proteins (D1 in blue, D2 in yellow, CP47 in violet, CP43 in brown; PsaA in blue with antenna helixes in light blue, and PsaB in ochre with antenna helixes in light ochre). (B) The PSII assembly goes on in a stepwise manner starting with the synthesis and insertion of precursor D1 module (pD1m) and continues with sequential attachment of D2, CP47 and CP43 modules. Formation of RCII complex is accompanied by CtpA protease-catalyzed maturation pD1, and it is supposed to be spatially separated into specific thylakoid membrane domains, so called biogenesis centres. Both the early and the later steps of PSII biogenesis are facilitated by specific assembly factors which are not components of the functional PSII.
A prominent representative of PSII-associated AFs is the protein Ycf48 (HCF136 in plants), which acts early in the biogenesis by binding to newly synthetized precursore D1 (pD1) and by promoting efficient association with D2 protein to form a PSII reaction centre assembly intermediate (RCII) (Komenda et al., 2008). Moreover, Ycf48 is required for efficient replacement of damaged D1 during the repair of PSII, and there is also evidence of a more wide-ranging role in the biogenesis of the photosynthetic apparatus. The Ycf48 forms seven-bladed beta-propeller with a conserved “arginine patch” on the surface of Ycf48 that is important for binding of Ycf48 to PSII RCs but also to larger complexes, including trimeric photosystem I (PSI) (Yu et al., 2018). In cooperation with our partners, we have recently solved a high resolution structure of the RCII formed by D1 and D2, PsbI and PsbE/F subunits and the attached Ycf48 protein (Zhao et al., 2023). Our results revealed that Ycf48 binds onto the lumenal surface of D1 through the Arg patch, at the site where the oxygen-evolving Mn cluster binds to D1 in mature PSII, thereby preventing the premature binding of Mn2+ and Ca2+ ions and in this way protecting the site from light-induced damage. Consequently, detachment of Ycf48 is an obligatory step for light-driven assembly of the Mn4CaO5 cluster. The model also indicated an interaction between Ycf48 and the C-terminal tail of D2 which helps explain how Ycf48 promotes assembly of the D1/D2 complex.
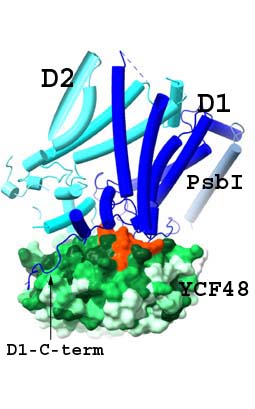 |
The early assembly factor Ycf48 (green) binds to D1 (dark blue) through the Arg patch (orange) at the site where the oxygen-evolving Mn cluster binds to D1 in mature PSII. The C-terminus of D1 (arrow), also involved in ligating the Mn cluster, wraps around Ycf48. This arragement prevents the premature binding of Mn2+ and Ca2+ ions and protects the site from damage. For more details see Zhao et al. (2023). |
Photosystem I assembly
In contrast to PSII, the detailed information on PSI assembly is much more limited and this concerns especially the early stage of the process. We have recently reached an important progress in the study of early phase of the PSI assembly by isolating individual FLAG-tagged PsaA and PsaB subunits from strains lacking the second member of the reactioin centre heterodimer (Gupta and Komenda, unpublished). The isolated PsaA contains a large number of Chls roughly corresponding to the PsaA-associated amount judged from the crystal structure of the functional trimer (Malavath et al. 2018). In contrast, the isolated FLAG-PsaB shows only very limited pigmentation. The presence of some small PSI membrane subunits speaks for the modular principle of assembly, which now seems to be common for both PSI and PSII. We also identified a number of specific proteins associated with each large subunit. Some of them were already known assembly factors but there were also previously unidentified components, potentially important for the early stage of PSI biogenesis. Our current project Early phase of PSI biogenesis .. is aimed at defining the detailed model of the early stage of PSI assembly and establishing the role of small PSI subunits and assembly factors identified in the early PSI assembly intermediates.
Intertwined biogenesis of PSI and PSII
The association of specific PSII intermediates with either monomeric or trimeric PSI (Bečková et al., 2017; Strašková et al. 2018; Kiss et al., 2019; Knoppová et al., 2022) or the enzymes of Chl-pathway (Dobáková et al., 2009 and unpublished results) as well as the association of Chl synthase, the last enzyme of Chl biosynthesis, with the YidC insertase and Sec translocase (Chidgey et al. 2014) indicate that the processes of biogenesis of the two photosystems probably share common cellular machinery integrating chlorophyll biosynthesis (see Intertwined biogenesis ..). We assume the existence of functional domains controlling the partitioning of de novo Chl molecules into PSI and PSII subunits to adjust PSI:PSII ratio under different environmental conditions, and we also suppose that the association of PSII assembly complexes with PSI protects them from photodamage due to energy spillover to PSI.
The role of FtsH proteases in quality control of photosynthetic complexes and the response to abiotic stress
FtsH proteases (FtsHs) belong to intramembrane ATP-dependent metalloproteases which we find in prokaryotes, mitochondria and chloroplasts. Most cyanobacteria, including Synechocystis, possess four FtsH homologs (FtsH1 to FtsH4). They assemble into three oligomeric complexes: two heterooligomers FtsH1/3 and FtsH2/3, and one homooligomer FtsH4. The FtsH1/3 complex is situated in the plasma membrane, while the more abundant FtsH2/3 and FtsH4 are localized in the thylakoid membranes. It has been shown that Synechocystis FtsH1/3 is crucial for fine-tuning the transcriptional nutrient stress response. On the other hand, the thylakoid FtsH2/3 governs the selective degradation of PSII subunits during the repair process, an essential mechanism compensating for PSII damage from excessive light, thus playing a crucial role in maintenance of active PSII complexes. Deletion of the ftsH2 gene results in abnormal light sensitivity and photoinhibition of PSII, and it also leads to a reduction in the PSI level and cellular Chl content. The other thylakoid FtsH4 complex is engaged in the biogenesis of PSII and PSI and acclimation to sudden changes in light intensity by regulating the level of high-light inducible proteins (Hlips) involved in photoprotection of the emerging PSII complexes. For a recent review see Krynická and Komenda (2024).
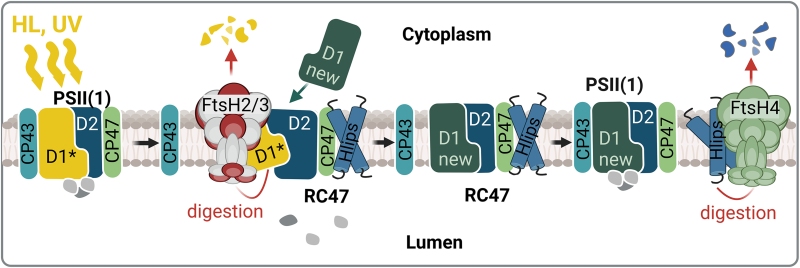
The role of the thylakoid FtsHs in photoprotection of the PSII complex. Under HL and UV stress, the fast inactivation of PSII is accompanied by the damage of the D1 protein (D1*) leading to the destabilization of CP43 binding within PSII. This destabilization allows access of FtsH2/3 to the damaged D1, which is then quickly degraded and replaced by the newly synthesized D1 (new D1). Subsequently, the CP43 antenna is reconnected to the repaired RC47. This repair of PSII occurs in the presence of high-light inducible proteins (Hlips), which protect PSII intermediates from oxidative damage by quenching the energy from the excited Chl. When the PSII repair is completed, the Hlips are released from the complex and their level is regulated by the FtsH4 protease (Krynická and Komenda, 2024).